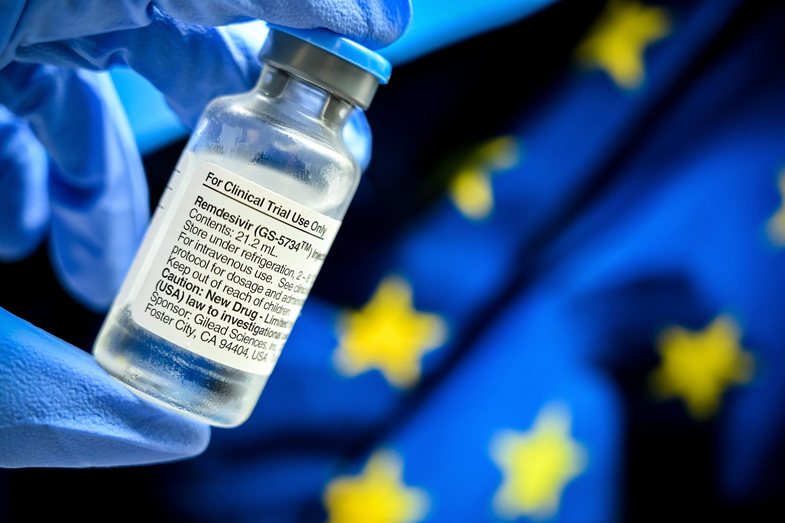
Remdesivir, one of the medications Donald Donald took when he became infected with Covid-19, should not be used in hospitals because there is no evidence that it works, the World Health Organization has advised. Donald Trump was one of the biggest supporters of remdesivir use. The WHO steering committee, however, has said that patients with Covid may be better off without it.
The WHO has published a recommendation against the use of remdesivir in hospitalized patients, despite the severity of the disease, as there is currently no evidence that remdesivir improves survival and other outcomes in these patients. This recommendation, issued today (November 20) is part of a guideline on clinical care for Covid-19 and was developed by an international group.
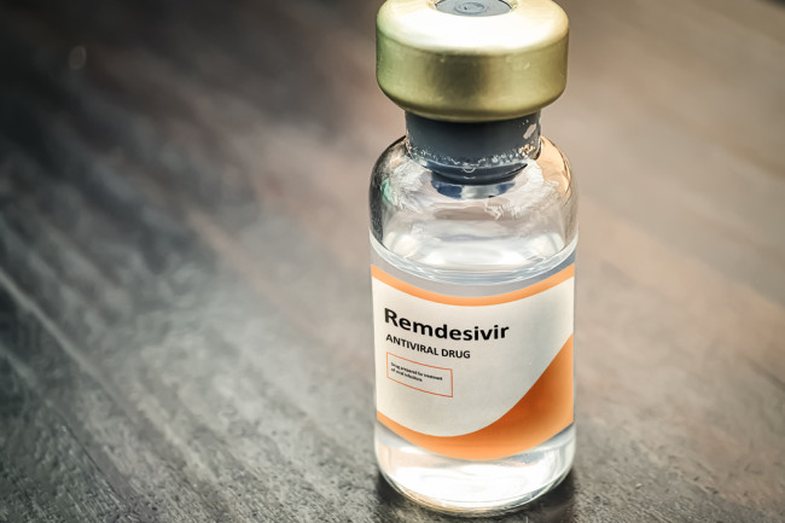
The US Food and Drug Administration (FDA) has authorized the use of remdesivir against Covid-19. Other countries use it as well. Manufactured by the American company Gilead, remdesivir is extremely expensive and must be given intravenously (by intravenous injection). Any beneficial effect of remdesivir, if any, is likely to be minor and there is a possibility of adverse effects.
Peter Horby, professor of infectious diseases and global health at Oxford University said the way remdesivir is being used needs to be reconsidered. He said tests performed on more than 7,000 Covid-19-infected adults found no evidence of any significant benefit from the drug.
- Fear of pandemic! Websites, apps and other strategies to relieve anxiety
- For how many days, people infected with Covid-19 can potentially transmit the virus
- The mutated coronavirus is reported in 7 countries: Good news and bad news
- Researchers give an explanation of the mystery of the loss of smell by Covid-19
- New Italian study: Covid-19 may not have originated in China, but earlier in Italy
Sources: WHO, Guardian, New York Times





