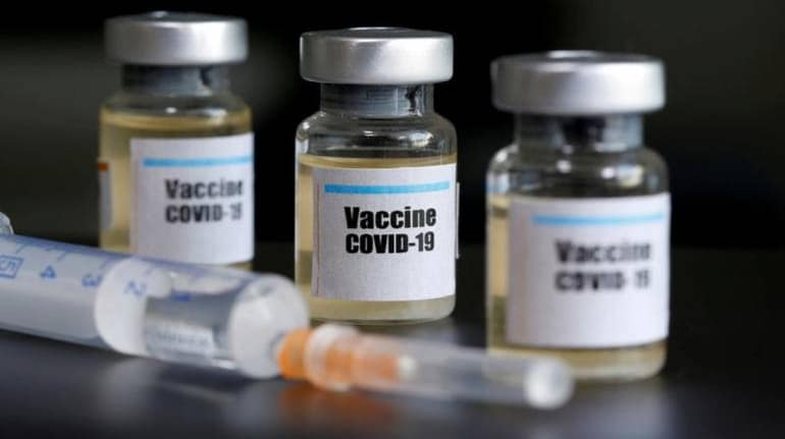
Just a week after Pfizer announced its vaccine against Covid-19, US pharmaceutical company Moderna said its vaccine in late-stage trials was 94.5% effective in preventing coronavirus. Happy Vaccine Month!
The Moderna trial tested 30,000 subjects and from the results it was found that the vaccine is almost 95% effective.
These results are still preliminary and not from a peer-reviewed study, but from an independent Moderna scoreboard review. Full results come out in late December.
Moderna's efforts have been part of Operation Sharp Speed, the US government's campaign to raise money for vaccines. They have already invested $ 2.5 billion in Moderna and purchased 100 million doses for U.S. citizens, according to Science.
The new vaccine is not identical to that of Pfizer, although both work by using the genetic code of the disease to trigger an immune response, according to the BBC.
The Guardian reports that the Moderna vaccine may be more effective when it comes to protection from a fallen Covid, as no person vaccinated in the Moderna trial had serious symptoms. Pfizer data does not specify this.
The Pfizer vaccine should be kept at -94F, but Moderna can be kept at refrigerated temperatures for a month and at room temperature for up to two hours, reports Sky News. This means it can be more durable and easier to transport.
The Moderna vaccine will also be more expensive. The Guardian reports that Moderna is being sold to the government for $ 50-60 for two vaccines, while Pfizer costs $ 40.
Moderna says the vaccine appears to work equally well across all racial and ethnic groups and is also effective in the elderly and people with certain medical conditions, such as diabetes, according to Science.
There is still no data on how vaccines would work in pregnant women.
As with the Pfizer-BioNTech vaccine, scientists do not know how long immunity from the Moderna vaccine will last.
The chief medical officer at Moderna, Tal Zaks, told the BBC that the vaccine "does not appear to be losing its potency" over time, but it's too soon to make sure.
No vaccine is available to the public yet because both need to complete their trials, which are expected to be completed by the end of December.
Source: Bustle