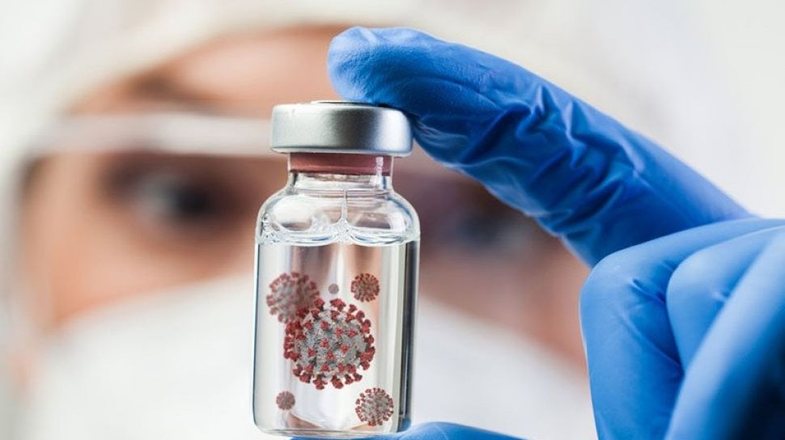
Johnson & Johnson has asked the US Food and Drug Administration (FDA) to approve its single-dose vaccine, which can be used to simplify the immunization campaign and prevent the virus from spreading more slowly.
The FDA application follows a Jan. 29 report in which the company - a multinational American corporation founded in 1886 that develops medical equipment, pharmaceuticals and packaged consumer goods - said the vaccine had a 66% rate of infection prevention in its global test.
J&J said it would also apply to European authorities for approval in the coming weeks.
"With the authorization of our Covid-19 vaccine for emergency use, we are ready to begin shipping," the company's scientific director, Paul Stoffels, said in a statement.
The FDA has meanwhile sought public debate over vaccine data after the first dose. During a recent conference, senior U.S. health official Dr. Anthony Fauci, said that the results of the Johnson & Johnson vaccine test mean that "now we have an additional candidate of added value of the vaccine".

Most vaccines used worldwide currently require two doses. But single-dose vaccines are significantly easier to distribute to populations, especially in areas where tracking people after the first dose can be a major challenge.
The J&J vaccine transports the "spike" gene into the body, where cells make harmless copies of proteins to fill the immune system in the event of a true virus. The vaccine developed by AstraZeneca and Oxford University works similarly but requires two doses.
Both AstraZeneca and J&J vaccines can be stored in an ordinary refrigerator, making them easier to transport and use in developing countries.

Sources: Guardian, Euronews